Doug Gisewhite presents research at the Northeast Regional Meeting of the American Chemical Society
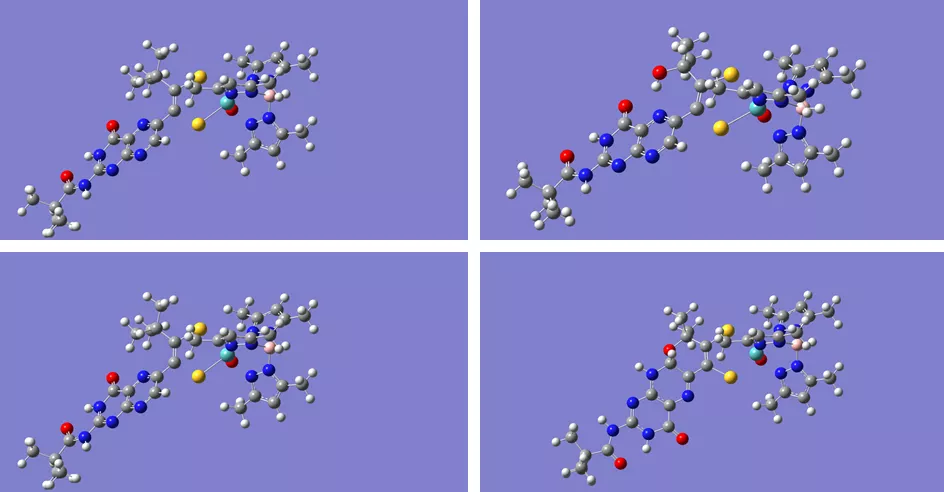
In June, Doug Gisewhite, PhD candidate in Chemistry, presented part of his ongoing research at the Northeast Regional Meeting (NERM) of the American Chemical Society (ACS) at Ithaca College. Doug's talk, "Molybdenum Pyranopterin Dithiolene Complexes: Synthetic Models for Pyran Cyclization in the Molybdenum Cofactor," detailed a new model system recently developed in Dean Burgmayer's lab.
Part of the team's research results were recently published in an article in the May 2015 issue of Organic Chemistry, "Solvent-Dependent Pyranopterin Cyclization in Molybdenum Cofactor Model Complexes." Doug expects to publish another article from new research he has been conducting since April in the Fall 2015 term.
"The Molybdenum Cofactor (Moco) plays a role in global carbon, nitrogen, and sulfur cycles. Moco always has a dithiolene moiety coordinating the molybdenum center to a pyranopterin. While the general structure is known, the role of the energetically expensive ligand (the pyranopterin ditholene) is not well understood. Difficulties probing the moiety in the biologically intact cofactor stem from the instability of Moco when isolated from the protein matrix. To overcome this issue, our group has developed a series of synthetic models to investigate the redox behavior of the ligand and it's effect on the metal center. Our published model systems exhibit a cyclization/scission process which is prevented in the new model. Prevention of the cyclization of the pyran ring may shed some light on why some biologically crystallized forms of Moco, such as nitrate reductase A, have two such ligands: one that is cyclized and one that is uncyclized."